“All public and private stakeholders engaged in this project in Estonia have understood the value this type of innovation can bring for the patients, the HCPs and the society. Estonia therefore united their unique technology availability with another critical success factor: joint willingness from all involved parties to collaborate. ”
The healthcare trend is to move towards the individual treatment of a person
Our healthcare systems collect information about human biology and clinical practice at an increasing rate. It has been estimated that by 2020 the available medical information doubled every three months. This flow of data is driving the healthcare shift from population-level to precision medicine. If we have enough information about people, then we can treat them individually, according to the unique traits of their medical histories, genomes and disease progressions. The outcomes of such treatment will create new data and improve treatments for the next patients.
However, this revolution in healthcare forces us to reimagine how we develop new drugs and treatments. Clinical development needs to move from an aggregated data model to a patient-level one. Shorter feedback loops mean that new medical knowledge has to reach clinical practice faster while keeping risks low and quality high. And all of this needs to comply with existing and upcoming ethics, privacy and artificial intelligence requirements such as the European General Data Protection Regulation (GDPR) and the AI regulation.

AI in healthcare panel discussion at Tallinn Digital Summit 2019, Cybernetica's Head of the Information Security Research Institute Dan Bogdanov third from right.
From health records to timely diagnostics
One dream application of personalised medicine way is to pick validated clinical markers or a diagnostic model and match them on health records held by healthcare providers or national health records. In the simple-yet-boring case, the analysis will result in a number of patients who could be affected by the disease. However, the impact of this is limited to policy changes.
GlaxoSmithKline (GSK) is a multinational pharmaceutical company developing a wide range of drugs and precision medicine initiatives. GSK has also developed drugs for very specific diseases like Severe Eosinophilic Asthma (SEA). While asthma is quite common, its severe eosinophilic form is rarer and does not respond as well to standard treatments. A specific biological treatment is needed, but first the doctors need to find, which of their patients need it. Testing everyone is not a cost-efficient option.
If the treating physicians could learn of a disease that they may not have diagnosed yet, it could improve the timeliness of treating the condition and avoiding complications which are expensive to manage and reduce years lived healthily.
In a breakthrough project, GlaxoSmithKline and Cybernetica have developed a blueprint for patient identification and intervention from national health records. With pulmonologists from the University of Tartu Hospital and the North Estonia Medical Centre providing the clinical validation, GlaxoSmithKline and Cybernetica designed a privacy-preserving clinical decision support tool that could find patients who might be suffering from Severe Eosinophilic Asthma by analysing the national health records.
A breakthrough project based on Privacy Engineering
Cybernetica used its privacy engineering methodology to plan both the trial and the patient identification tool so that they would use an absolutely minimal amount of personal data. Cybernetica's engineers picked the best points for deploying privacy technologies like de-identification and the Sharemind HI confidential computing system, so the risk of patient re-identification or intellectual property leakage would be minimal.
In parallel, GSK worked with pulmonologists, legal experts and the Ministry of Social Affairs to put together the medical validation methodology and understand how such a service could be operated within the Estonian healthcare ecosystem. Also, GSK worked with Cybernetica and a third-party security team to validate Cybernetica's security claims about the tool design and the use of Sharemind HI in it.
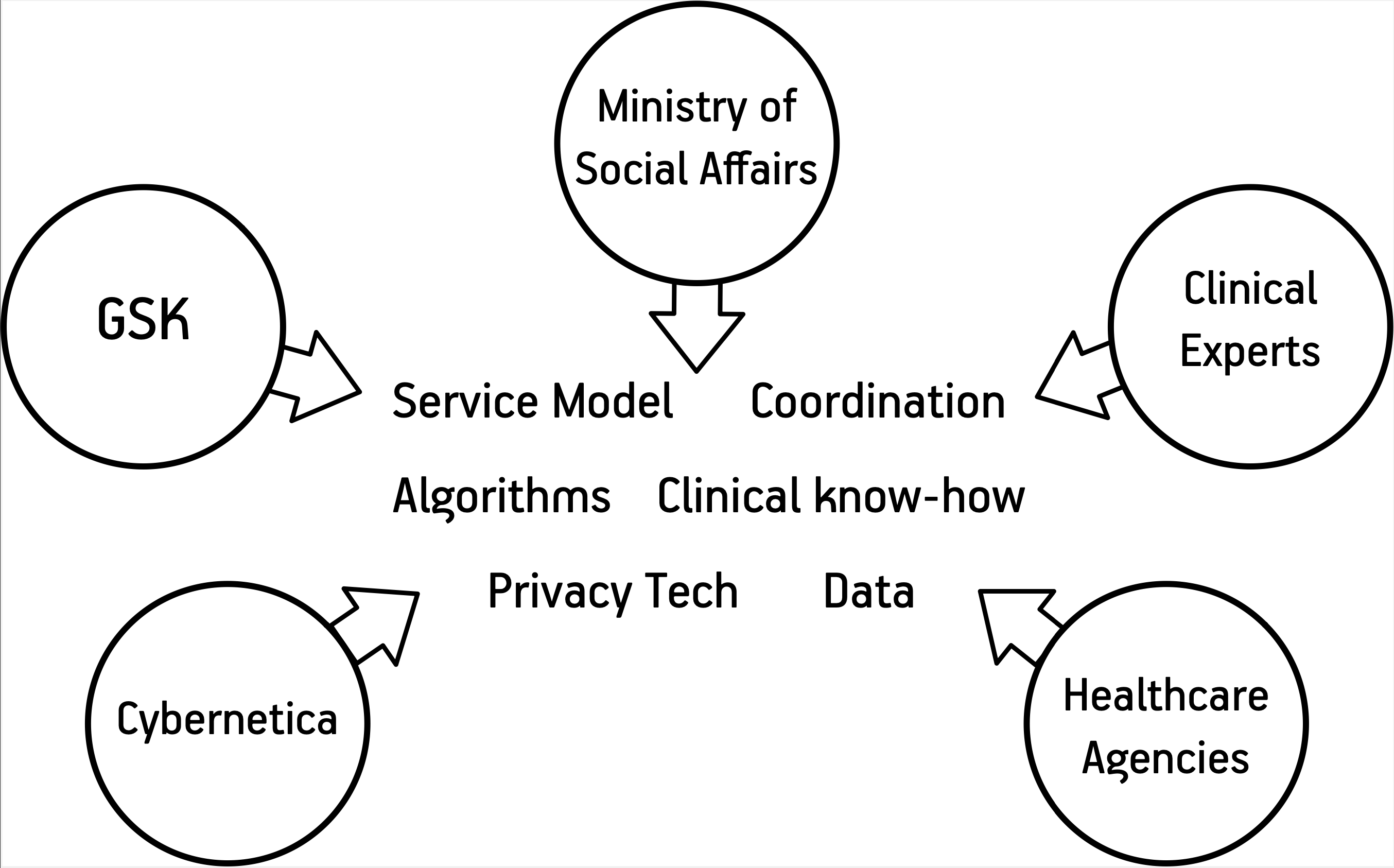
“Estonia has an established digital health infrastructure with > 95% of healthcare information digitised. The availability of digital and connected information through patient databases, electronic medical records (EMC), as well as prescribing and dispensing data, provides an unmatched opportunity to develop an innovative approach to supporting healthcare providers to improve outcomes. This project was not a diagnostic tool. It was intended to support HCPs in their detection & diagnosis of this rare form of asthma. As new and more specialised treatments arise, addressing the needs of small patient populations and where the number of patients in a country is much lower than the number of treating family doctors, the question of patient identification and referral for diagnosis & advice needs to be answered. Cybernetica played a major coordination role in this project that GSK was sponsoring, thanks to their unique expertise of the technology, the Estonian environment and ways of working and their focus on data protection and privacy," said Georges Dagher, General Manager of GSK Baltics.
Cybernetica produced two designs in the project. The first combined security and privacy techniques to validate the patient identification system in a research setting. The design was evaluated and confirmed by the Estonian Bioethics and Human Research Committee. The second design was for deploying patient identification with end-to-end security using Sharemind HI.
What next?
The new design creates a new go-to-market strategy for drugs, treatments and new forms of personalised medicine services. Cybernetica’s Sharemind privacy-preserving data analysis technology connected the dots and resolved several pain points that pharmaceutical companies have been tackling over the years.
